Advancing a Prototype POC Cartridge to Commercial-Ready Manufacturing
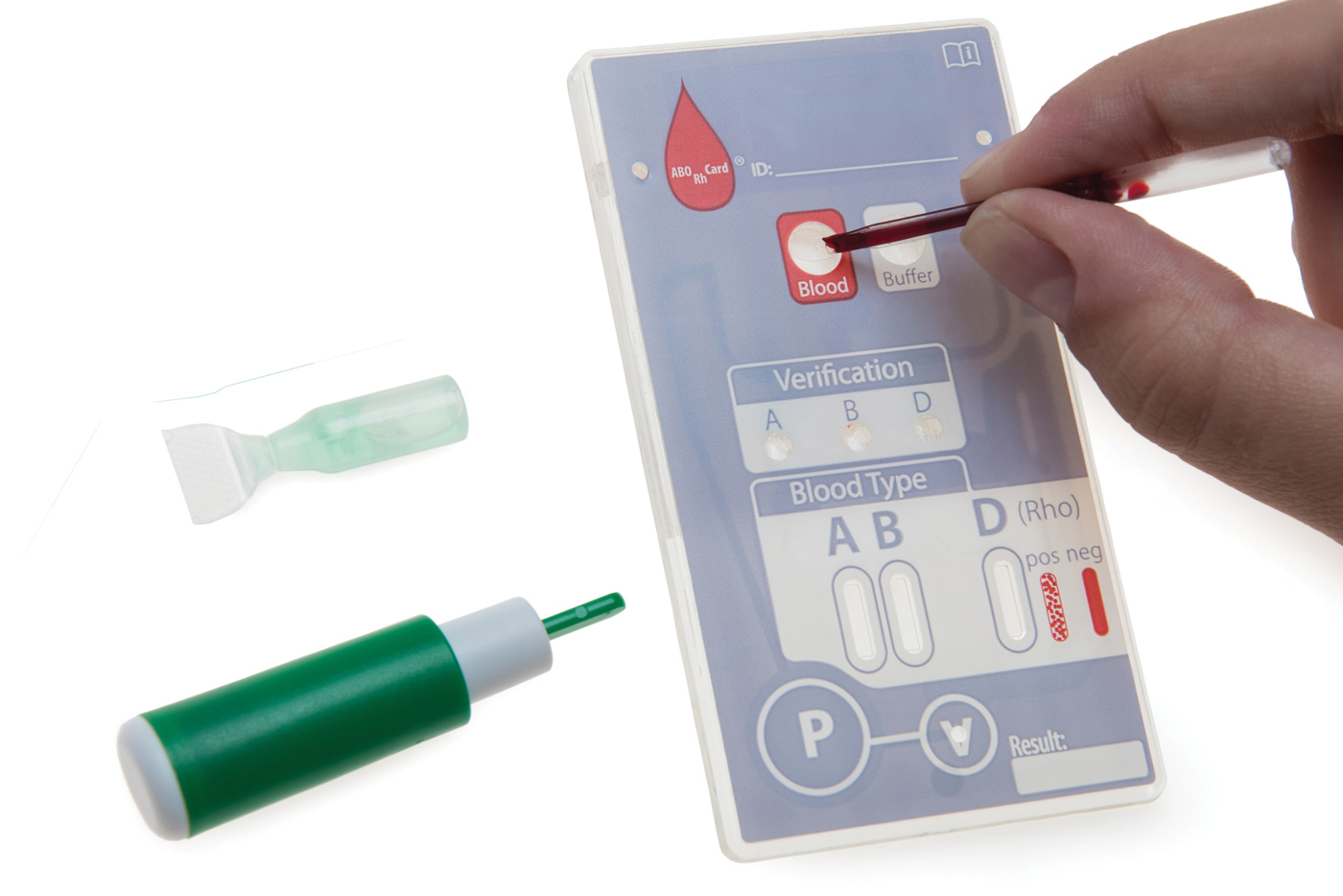
Case Study: Transitioning a Field Blood Typing Card from Prototype to Commercial Scale
Overview
SMC collaborated with a major OEM to transform a prototype point-of-care (POC) blood typing cartridge into a fully commercialized product. This program required transitioning early-stage, prototype-level processes into robust, scalable, and validated manufacturing workflows capable of meeting growing market demand.
The Challenge
Successfully commercializing the Field Blood Typing Card required overcoming a range of technical, environmental, and scale-up challenges:
- Transferring prototype processes and equipment into validated, production-ready systems
- Molding highly precise microfluidic channels in polycarbonate
- Ensuring accurate, repeatable application of micro-volume fluid control
- Managing environmental conditions for accurate spotting of cards
- Developing a manufacturing plan capable of scaling with rapidly increasing demand
The Solution
SMC engineered a complete manufacturing pathway to support commercial readiness, integrating chemistry, tooling, automation, and quality systems. Key elements included:
- Integrating chemistry formulation and handling directly within the manufacturing process
- Meeting stringent microfluidic requirements via specialized tooling and molding processes
- Developing custom dispensing systems with highly precise formulation control
- Creating and executing task-specific validation protocols
- Establishing a robust QMS and automated labeling systems across the full BOM
- Designing custom spotting equipment with tightly controlled environmental parameters
- Building a scalable staffing, equipment, and production plan to meet market growth
The Results
Through disciplined process development, tooling expertise, and integrated chemistry handling, SMC successfully delivered a commercial-ready field blood typing consumable. The collaboration resulted in:
- A fully validated, production-ready version of a previously prototype-only POC device
- Consistent and reliable microfluidic performance enabled by precision tooling
- Greater accuracy and repeatability through customized dispensing and spotting systems
- Strong quality control with automated labeling and comprehensive validation
- A scalable, flexible manufacturing platform aligned with market expansion